Share
Over the last 25 years we have changed our thinking about glaucoma – whereas once it was considered to be a degenerative disease of aging, we now view glaucoma as a complex genetic disease. Consequentially, this has changed our screening and management process. Family history now plays a key part in identifying high risk individuals, and genetic testing is rapidly broadening as new genes are discovered. With over 100 genes that cause glaucoma or raise intraocular pressure (IOP) identified, we hope to soon have a genetic test for people who have a family history of glaucoma, and eventually for the entire population.

Glaucoma is one of the most heritable common diseases. Indeed, two very large studies in the United States, using health insurance and claims data, have shown glaucoma to be one of the most heritable of all types of diseases.1,2 Studies such as the UK Biobank have identified over 100 genes for Mendelian and complex forms of glaucoma.3,4,5
In Australia, with the support of Glaucoma Australia (GA, previously The Glaucoma Foundation of Australia), researchers have been undertaking studies on glaucoma genetics since the discovery of the GLC1A gene in 1993.
GA has supported the Glaucoma Inheritance Study in Tasmania (GIST) since its early days in 1994 by providing funding, recruiting glaucoma patients for the study, and by developing clinic posters (Figure 1. A Glaucoma Australia poster from the mid 1990s.).
The population of Tasmania is ideal for genetic research because of its good genealogy records due to a founder effect (where many people are descended from early immigrants to the island) and lower levels of recent immigration compared to the rest of Australia. In the 1940s, National Health and Medical Research Council (NHMRC) funded research into genetic eye disease in Tasmania revealed large pedigrees with other hereditary eye diseases, due to hundreds of carriers who descended from a single immigrant in the convict era.6 At that time, few glaucoma pedigrees were identified because most cases were undiagnosed until people went blind from the disease. Additionally, few patients informed their relatives they were being treated for glaucoma.
 The genetic research undertaken in Tasmania has been invaluable to building our knowledge of the genes associated with glaucoma. The GIST study was completed with the cooperation of all Tasmanian ophthalmologists, many local optometrists, general practitioners, and pharmacists who enrolled in glaucoma cases, determined ancestry, and used Tasmanian genealogical records to connect glaucoma patients into pedigrees. Between 1994 and 1999, nearly 2,000 cases and 3,000 relatives were examined.
The genetic research undertaken in Tasmania has been invaluable to building our knowledge of the genes associated with glaucoma. The GIST study was completed with the cooperation of all Tasmanian ophthalmologists, many local optometrists, general practitioners, and pharmacists who enrolled in glaucoma cases, determined ancestry, and used Tasmanian genealogical records to connect glaucoma patients into pedigrees. Between 1994 and 1999, nearly 2,000 cases and 3,000 relatives were examined.
This important study helped identify the Myocilin gene and severity of the disease, which varied with different mutations (phenotype-genotype correlations).7
It also found that over 40 per cent of people have a first degree relative (parent, brother or sister) affected by glaucoma.8
Importantly, GIST showed at that time, over a quarter of people were unaware of their family history of glaucoma – even those with a strong family history.9 Additional glaucoma genes10 were identified by the Australian and New Zealand Registry of Advanced Glaucoma (ANZRAG – www. ANZRAG.com),11 using genome-wide association studies (GWAS).
Family history data from the GIST helped inform the National Health and Medical Research Council (NHMRC) guidelines for Screening, Prognosis, Diagnosis, Management and Prevention of Glaucoma. These guidelines included recommendations for screening high risk individuals such as first degree relatives (siblings and children) of glaucoma patients, who are at 10 times greater risk of glaucoma than the general population.12
Awareness Increases Screening
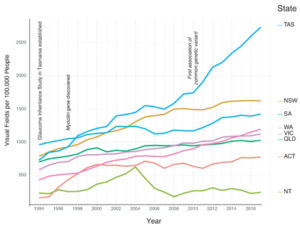
Medicare data available from 1994 through 2018 for visual field testing, which is most often performed to test for glaucoma, shows that GIST research and the NHMRC guidelines developed as a result of its findings, have significantly impacted glaucoma screening in Tasmania.
Data shows a quadrupling in the number of visual field tests conducted in Tasmania (from 733 services per 100,000 population to 2,732 per 100,000 population) compared with only a doubling nationally (Figure 2).6
Part of this increase in Tasmania was due to the aging population, with the island state experiencing the largest increase in median age. However, even adjusting for the increased number of Tasmanians over the age of 40, there was still a large increase in the number of visual field tests conducted. This was likely due to the enhanced awareness of the need for screening among the thousands of at-risk members of the glaucoma families in Tasmania and their health providers. The next highest rates of visual field testing are in New South Wales and South Australia, which have the highest enrolments in the ANZRAG. With this evidence in mind, health professionals should encourage glaucoma patients to inform their family members to seek clinical screening.
Identifying Glaucoma Genes
Identifying additional genetic markers for glaucoma will continue to grow our understanding of the disease and its progression, enabling us to better differentiate those at low risk from those at high risk and manage them accordingly. This will be achieved by expanding the invaluable pilot work conducted in Tasmania across Australia, thanks to an NHMRC partnership grant (Targeting at risk relatives of glaucoma patients for early diagnosis and treatment (TARRGET) study).13
 Ongoing work to identify the genetic markers for glaucoma will also be undertaken via a NHMRC program grant (Translating genetic determinants of glaucoma into better diagnosis and treatment), and collaborations with the International Glaucoma Genetics Consortium (IGGC). This work will allow us to differentiate family members who need ongoing examinations from those who can be discharged from regular follow up. Although screening all high risk relatives may increase the costs of surveillance, this should be recouped by costs saved due to reduced blindness.
Ongoing work to identify the genetic markers for glaucoma will also be undertaken via a NHMRC program grant (Translating genetic determinants of glaucoma into better diagnosis and treatment), and collaborations with the International Glaucoma Genetics Consortium (IGGC). This work will allow us to differentiate family members who need ongoing examinations from those who can be discharged from regular follow up. Although screening all high risk relatives may increase the costs of surveillance, this should be recouped by costs saved due to reduced blindness.
Cascade family and genetic screening protocols are being refined, which should allow us to prevent blindness, maintain quality of life, and conserve resources. If we can identify family members at low risk for glaucoma by using genetic screening, we can reassure them and reduce their need for expensive clinical screening. This will also allow us to focus our resources on those at high risk.
Understanding the Biology of Glaucoma
In addition to helping identify those people at risk for glaucoma, newly identified genes are increasing our understanding of the biology of glaucoma and opening up potential new glaucoma treatments.
Analyses of the UK Biobank have shown new pathways that are involved in both paediatric and adult forms of glaucoma. Many of the loci identified as associated with increased intraocular pressure (IOP) or primary open angle glaucoma (POAG) are now known to be associated with other eye conditions. LTBP2 has been found to cause primary congenital glaucoma. TEK has been associated with primary congenital glaucoma, and we identified common IOP influencing variants in genes that interact with TEK (ANGPT1, ANGPT2 and ANGPTL2). Anterior segment dysgenesis, iris abnormalities, nanophthalmos, and microcornea are known causes of secondary glaucoma.
In the UK Biobank study, four genes influencing IOP in the general population have been associated with anterior segment dysgenesis or other abnormalities of the iris, lens, or cornea: FOXC1 with ocular anterior segment dysgenesis; TRAF3IP1 with iris furrows; MFRP with nanophthalmos; and ADAMTS18 with microcornea, myopic chorioretinal atrophy and telecanthus. Another – LMX1B – led to nail patella syndrome; common variants at this locus are now definitively associated with both POAG and IOP. Interestingly, three loci (PLEKHA7, FERMT2 and GLIS3) that were previously associated with primary angle closure glaucoma (PACG), are now implicated with IOP; two of those regions (PLEKHA7 and FERMT2) also showed association with POAG.
Ongoing work with the International Glaucoma Genetics Consortium will identify more of the genes which influence glaucoma risk. Given the strong genetic basis for glaucoma, in the future genetic testing for glaucoma will become increasingly accurate, and gene-based screening will help reduce the number of Australians blinded by the disease.
Professor David Mackey is the chair of Ophthalmology at the University of Western Australia and the former Managing Director of the Lions Eye Institute in Perth. He is a former councillor of the Royal Australian and New Zealand College of Ophthalmologists (RANZCO) and current RANZCO representative on the Council of the Asia Pacific Academy of Ophthalmology. He is past president of the International Society for Genetic Eye Disease and Retinoblastoma. Prof. Mackey is a renowned international researcher in the genetics of eye disease and has published over 340 peer reviewed papers since 1989. He is the world’s most published author in glaucoma genetics and is a lead investigator in the International Glaucoma Genetics Consortium (IGGC) and the Consortium for Refractive Error and Myopia (CREAM). In 1993 Professor Mackey initiated the Glaucoma Inheritance Study in Tasmania (GIST), thereby creating one of the largest glaucoma biobanks in the world, with over 5,000 DNA samples and clinical material from familial and sporadic cases of glaucoma.
Professor Jamie E Craig is Chair and Academic Head of the Department of Ophthalmology (ERA rank 5) at Flinders University and a Consultant Ophthalmologist. He specialises in the care of patients with glaucoma and is a clinician-scientist and NHMRC Practitioner-Fellow, who translates laboratory based research into clinical practice.
Professor Alex W Hewitt is a clinician-scientist who completed a B.Med.Sci.(Hons) degree investigating the outcomes of cataract surgery for people living in remote areas of the Northern Territory, and his undergraduate medical degree at the University of Tasmania. He obtained his PhD investigating the molecular and phenotypic associations for open angle glaucoma from Flinders University of South Australia in 2009 under the supervision of Professors Jamie Craig and David Mackey. Prof. Hewitt completed formal ophthalmology training at the Royal Victorian Eye and Ear Hospital in Melbourne in 2011, and the following year he was the Novartis Research Fellow at the Lions Eye Institute and was awarded a WA Tall Poppy Award from the Australian Institute of Policy & Science.
Associate Professor Stuart Macgregor is a statistical geneticist, who studies the role genetic variation plays in determining risk of disease and its risk factors. His Statistical Genetics laboratory develops and applies statistical genetic methods to gene mapping studies across a wide range of traits and diseases. Assoc/Prof. Macgregor’s work to date has focused on development of statistical genetic methods and on their application to disease. In these areas he has published over 100 research articles since 2002, frequently in top journals.
References
- Wang K, Gaitsch H, Poon H, Cox NJ, Rzhetsky A. Classification of common human diseases derived from shared genetic and environmental determinants. Nat Genet. 2017;49(9):1319-25.
- Femanda CG, Polubriaginof FCG, Vanguri R et al. Disease Heritability Inferred from Familial Relationships Reported in Medical Records. Cell. 2018 Jun 14;173(7):1692-1704.e11.
- Khawaja AP, Cooke Bailey JN, Wareham NJ, et al. Genome-wide analyses identify 68 new loci associated with intraocular pressure and improve risk prediction for primary open-angle glaucoma. Nat Genet. 2018 Jun;50(6):778-782
- Gao XR, Huang H, Nannini DR, et al. Genome-wide association analyses identify new loci influencing intraocular pressure.Hum Mol Genet. 2018 Jun 15;27(12):2205-2213.
- MacGregor S, Ong JS, An J, et al. Genome-wide association study of intraocular pressure uncovers new pathways to glaucoma. Nat Genet. 2018 Aug;50(8):1067-1071
- Mackey DA, Craig JE, Hewitt AW. Seeing the impact of the Glaucoma Inheritance Study in Tasmania after 25 years. Clin Exp Ophthalmol. 2018 Nov 22. doi: 10.1111/ceo.13446. [Epub ahead of print]
- Craig JE, Baird PN, Healey DL, McNaught AI, McCartney PJ, Rait JL, et al. Evidence for genetic heterogeneity within eight glaucoma families, with the GLC1A Gln368STOP mutation being an important phenotypic modifier. Ophthalmology. 2001;108(9):1607-20.
- Green CM, Kearns LS, Wu J, Barbour JM, Wilkinson RM, Ring MA, et al. How significant is a family history of glaucoma? Experience from the Glaucoma Inheritance Study in Tasmania. Clin Exp Ophthalmol. 2007;35(9):793-9.
- McNaught AI, Allen JG, Healey DL, McCartney PJ, Coote MA, Wong TL, et al. Accuracy and implications of a reported family history of glaucoma: experience from the Glaucoma Inheritance Study in Tasmania. Arch Ophthalmol. 2000;118(7):900-4.
- Burdon KP, Macgregor S, Hewitt AW, Sharma S, Chidlow G, Mills RA, et al. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat Genet. 2011;43(6):574-8.
- Souzeau E, Goldberg I, Healey PR, Mills RA, Landers J, Graham SL, et al. Australian and New Zealand Registry of Advanced Glaucoma: methodology and recruitment. Clin Exp Ophthalmol. 2012;40(6):569-75.
- nhmrc.gov.au/sites/default/files/201810/cp113_b_ glaucoma_guide_healthcare_workers_120404.pdf
- Staffieri SE, Ruddle JB, Kearns LS, Barbour JM, Edwards TL, Paul P, et al. Telemedicine model to prevent blindness from familial glaucoma. Clin Exp Ophthalmol. 2011;39(8):760-5.


